Name:______________________ Date:______________________
Worksheet 3. Basic Ionic Structure
[Back
To Unit 2]
Fill in the information missing from the table below.
|
Name
|
Symbol
|
Proton #
|
Electron #
|
Neutron #
|
Charge
|
| iron
(III) |
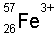
|
26
|
23
|
31
|
3+
|
| sulfide
ion |
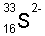
|
16
|
18
|
17
|
2-
|
| fluoride |
|
|
|
|
1-
|
| potassium ion |
|
|
|
|
1+
|
| |
|
7
|
|
7
|
3-
|
| copper (II) |
|
|
|
34
|
|
| |
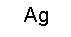
|
|
|
61
|
1+
|
| |
|
|
10
|
8
|
2-
|
| iron (II) |
|
|
|
31
|
|
Notes:
-
For this exercise, DO NOT include the mass number as part of the ion name.
-
A metallic cation will include a Roman numeral as part of its name if it
is formed from an element which can form two or more differently charged
ions. The Roman numeral gives the positive charge of the specific ion intended.
Transition metal ions almost always have charge indicated by Roman numerals.
Metallic elements in periodic table columns 1A and 2A form only one ion
and thus do not require charge specification as part of the name.
-
An ion formed from a single nonmetal atom is given a name derived from
the name of the element but ending in ide.